BIRLA CARBON BLOG
INSIDER KNOWLEDGE FOR ALL THINGS CARBON BLACK
Improving the Performance of Lithium ion Batteries with Conductive Carbons
09 / 14 / 2021 by Dr. Michael Regula and Dr. Zachary Combs
Reading Time: 3.5 minutes
First commercialized in the early 1990s, lithium ion batteries have since come to be an integral part of modern life. Portable electronic devices, such as cell phones, laptops, and power tools, were the first to utilize this technology. In recent years, the push to electrify personal transportation has accelerated the production of electric vehicles. Batteries for electric vehicles must achieve rigorous performance and economic metrics, including the following:
- Safety – Batteries must be able to charge and discharge without short-circuiting and thermal runaway.
- Energy Density – A high energy density is needed to achieve a driving range comparable to an internal combustion engine.
- Fast Charging – A fast charging rate without material degradation is needed to achieve fill-up times comparable to filling a petrol tank.
- Cycle Life – Delivering repeatable performance over many cycles improves both the economics and sustainability of batteries.
- Cost – The cost for producing a battery pack needs to fall below $100/kWh to achieve price parity with internal combustion engines.
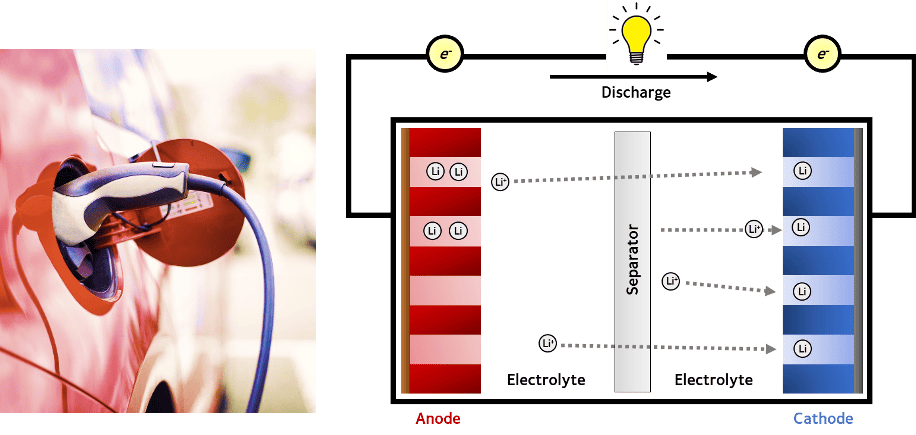
Charge storage in lithium ion batteries occurs using unique chemistry. The active materials in lithium ion batteries are typically layered structures. These layered structures have enough space between them to allow lithium ions to insert in between the layers, a mechanism known as “intercalation”, as shown below. Intercalation is a highly reversible process which allows lithium ion batteries to deliver high energy density for over thousands of cycles. Lithium ion batteries, therefore, work by shuttling the lithium ion between the layered structures of the cathode and anode active materials.
Extensive materials engineering and formulation development have been conducted for lithium ion batteries to become a commercial success. Lithium ion battery formulation typically consist of three components: an active material, a conductive additive, and a binder. Despite making up less than 5 wt% of typical lithium ion battery formulations, the conductive additive is critically important for maximizing the energy density and rate capability of the active materials.
Carbon blacks are the most widely used conductive additives because they can produce robust electrical networks in the electrodes. These electrical networks are best achieved by carbon blacks that have a high structure and extensive branching. By coating the active material particles and filling in the interstitial space in the electrode with a conductive carbon black, the kinetics of the intercalation reactions of both the cathode and the anode can be greatly improved. At Birla Carbon, our expertise in microscopy allows us to visualize carbon black’s role in lithium ion battery electrodes, as shown in the scanning electron microscopy (SEM) image below.
In addition to structure and branching, the surface area of the conductive additive is also important to consider when making material selections for battery formulations. Higher surface area materials generally have more reaction sites. In the early stages of battery cycling, the components of the liquid electrolyte – the medium through while lithium ions move between the electrodes – degrade at these reaction sites.
The degraded electrolyte forms a thin film known as the solid electrolyte interface (SEI). While lithium is lost during SEI formation, the SEI ionically conducts lithium to the active materials and protects the active material particles from degradation during cycling. If the SEI layer is too thick, though, the diffusion of the lithium ions through the SEI can be hampered and negatively affect battery performance. Structure and branching of the carbon black are generally the properties most well correlated to battery performance, but surface area also must be considered to ensure efficient battery cycling.
Conductive carbon black additives can also have a significant impact on the properties of both anode and cathode formulation. In traditional lithium ion battery formulations, where the active material, conductive additive, and binder are mixed into a viscous slurry, the carbon black must be well dispersed in that slurry. High structure carbon blacks demonstrate easier dispersion. The dispersibility of the carbon black can have a profound effect on the viscosity and achievable solids loading of the slurry. Slurries with a higher solids loading use less solvent, which can greatly improve the efficiency of battery production.
Birla Carbon has developed new conductive additives for use in both anodes and cathodes for lithium ion batteries. These conductive additives include high structure carbon blacks and carbon nanotube/carbon black hybrids that can push the boundaries of energy density, power density, charging rates, and cycle life. Formulations with Birla Carbon’s conductive additives also allow for increased manufacturing efficiency.
Contact EnergySystems@adityabirla.com to inquire about our exciting new conductive additives for lithium ion batteries.
Dr. Michael Regula
Dr. Michael Regula is an Engineer for Energy Systems at Birla Carbon with a primary focus on the development and characterization of new products and processes for the energy storage market. Research and development interests include synthesizing and characterizing carbon conductive additives, including nanomaterials, and active materials for both lithium ion batteries and next generation energy storage technologies. He is also focused on developing methods to define unique structure-property relationships to better engineer battery materials. Dr. Regula received his bachelor’s degree and PhD in chemical engineering from Penn State University. His PhD projects focused on developing active materials and electrolytes for next generation lithium-based batteries.
Dr. Zachary Combs
Dr. Zachary Combs is the Global Technology Leader for Energy Systems at Birla Carbon. He has more than 8 years of experience in the carbon black industry with a primary focus on the development of new products for the energy storage market and extensive past experience in tire and mechanical rubber goods industries. Research and development interests include the morphology, surface modification, and microstructure of carbon blacks and other advanced carbon materials, and how these characteristics impact structure-property relationships of energy storage systems. Dr. Combs received his bachelor’s degree in polymer chemistry from Clemson University and his PhD from Georgia Institute of Technology in materials science and engineering with a focus on nanoparticle synthesis and surface chemistry.
WANT TO LEARN MORE ABOUT CARBON BLACK AND THE PROPERTIES THAT MAKE IT IDEAL FOR YOUR PLASTICS, COATINGS, AND INKS APPLICATIONS?











Leave A Comment